Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua.
Panel Discussion on Biotech-CDMO Collaboration
Beyond partnerships: redefining CDMO-biotech collaboration in the age of complexity
Operational Chemistry: Making Biotech–CDMO Teams Actually Work
Commentary article

Kishore Hotha, PhD, MBA
President, Dr. Hotha’s Life Sciences LLC
Dr. Kishore Hotha is a distinguished leader in the pharmaceutical biotech and CDMO sectors, with a strong track record in advancing drug substance and product development across small and large molecules, including Antibody-Drug Conjugates (ADCs), oligonucleotides, peptides, and complex formulations. Throughout his career, he has been pivotal in the submission of numerous INDs, NDAs, and ANDAs, guiding these projects from concept to commercialization. Currently, Dr. Hotha is the President of Dr. Hotha’s Life Sciences LLC, a consulting firm dedicated to simplifying complex drug development challenges. With over 100 publications and several editorial board positions, Dr. Hotha remains an influential figure in shaping industry standards and advancing pharmaceutical innovation.
Introduction
As the landscape of therapeutic development rapidly evolves, so do the operating models that support it. The relationship between biotechs and Contract Development and Manufacturing Organizations (CDMOs) has fundamentally shifted—from transactional outsourcing arrangements to strategic co-execution frameworks. In an era marked by increased scientific complexity, shorter Funding cycles, and increased regulatory scrutiny, there is no room for misalignment.
Advanced modalities such as antibody-drug conjugates (ADCs), oligonucleotides, and mRNA-based therapies require seamless integration across analytical development, quality systems, regulatory readiness, and manufacturing execution. At the same time, policies like the BioSecure Act highlight the need for operational transparency, supply chain traceability, and mitigation of geopolitical risks.
Despite these changes, many collaborations between biotechs and CDMOs still face issues like unclear expectations, fragmented decision-making, and reactive project management. Biotechs often enter partnerships expecting faster results and greater flexibility, while CDMOs struggle to interpret vague scopes within tight timelines—leading to operational conflicts, missed milestones, and loss of trust.
To better understand how collaboration models can be improved, this commentary relies on structured input from multiple biotech and CDMO leaders across the industry. Their responses to a curated set of operational and scientific questions highlight key patterns in both failure and success.
Specifically, this panel discussion explores:
- How biotechs assess CDMO fit for complex modalities
- How CDMOs engage with limited early-phase data to de-risk scale-up
- What practices foster trust, alignment, and decision clarity
- And how both sides can shift from coordination to genuine co-execution
The goal is not to repeat familiar challenges, but to suggest a practical operating framework for CDMO–biotech partnership—one rooted in mutual accountability, scientific consistency, and behavioral unity. In a sector where the pace of innovation now depends on operational discipline, collaboration has become a key skill.
From Vendors to Velocity Partners: Reframing CDMO–Biotech Collaboration
The conventional construct of the CDMO–biotech relationship — a transactional outsourcing model centered on deliverables and timelines — is no longer tenable in today’s high-stakes, modality-driven development environment. The increasing complexity of drug candidates such as antibody-drug conjugates (ADCs), oligonucleotides, and RNA-based therapeutics, combined with compressed development cycles and evolving regulatory expectations, demands a new framework: strategic co-execution.
In this model, CDMOs are not external executors but integrated contributors — scientific, operational, and regulatory partners accountable for shared success. The transition from “vendor” to “velocity partner” is not semantic. It is a fundamental redefinition of how biotech companies must approach outsourcing if they are to accelerate development without compromising quality or compliance.
Moving Beyond Siloed Execution
Legacy engagement models — often driven by procurement timelines or minimal technical criteria — fail to anticipate the ambiguity and risk that define early- and mid-phase development. Under such conditions, misalignment between sponsor expectations and CDMO operations leads to common and costly issues: rework, scope creep, tech transfer delays, and strained regulatory interactions.
Conversely, high-functioning partnerships exhibit the following characteristics:
- Early-stage technical integration involving cross-functional input from chemical, finished dosage, analytical, quality, regulatory, and manufacturing teams.
- Bidirectional accountability, where both parties share responsibility for meeting milestones and managing risk.
- Operational transparency, backed by shared project governance and real-time data access
- Unified interpretation of priorities, particularly around speed, quality, and cost under constraint
These are essential attributes for ensuring reliable execution amidst the rising demands of fast-to-clinic programs, not just aspirational ideals.
Values as Decision Infrastructure
Building on our previous work on organizational coherence, it is clear that values are more than just soft concepts; they serve as practical decision-making frameworks. When timelines are tight and information is limited, what guides CDMO and biotech team behavior? Is there a shared understanding of what to prioritize, when to escalate issues, and how to interpret “client-centric” or “risk-based” approaches?
Many breakdowns stem not from capacity or capability gaps, but from behavioral misalignment under stress. A CDMO may view “flexibility” as last-minute rework; a biotech might see it as proactive problem-solving. Without a shared language and decision logic, trust diminishes.
Effective CDMO–biotech collaborations, therefore, require aligned principles, not just aligned timelines. These principles must be embedded into operating rhythms — kickoff templates, governance models, risk registers — and reinforced in moments of ambiguity.
From Tactical Execution to Strategic Orchestration
CDMOs that succeed in this new paradigm act as orchestration partners. They proactively anticipate downstream regulatory implications, incorporate phase-appropriate controls, and guide biotechs through uncertainties. This role requires scientific fluency, regulatory literacy, and behavioral maturity to share ownership effectively.
Biotechs, in turn, must shift from outsourcing activity to co-designing execution — embracing clarity, readiness, and communication as core enablers, not overhead. CDMO–biotech collaboration must evolve from a supplier-based relationship to a strategic execution model grounded in alignment, transparency, and mutual accountability. This evolution is not optional. It is the price of speed, and the currency of trust in a biotech landscape defined by complexity and consequence.
What Biotechs Must Get Right: Clarity as a Competitive Advantage
While much focus is on CDMO performance and technical skills, a collaboration’s success or failure often starts earlier—in how biotechs prepare, set, and manage their own expectations. From the feedback collected from CDMO leaders across the panel, a clear pattern appeared: inefficiencies in early phases and problems in later execution are often caused by a lack of clarity from the biotech sponsor.
Precision Begins with the RFP
The request for proposal (RFP) sets the tone for the partnership, yet many CDMOs report that biotech RFPs are often aspirational rather than actionable. Common gaps include:
- Unclear project scope
- Incomplete analytical or process characterization data.
- Misaligned timelines relative to technical complexity
- Omission of phase-appropriate regulatory context
A robust RFP should clearly articulate not only the “what” (deliverables) but also the “why” (clinical objective, development phase, regulatory intent) and the “how” (critical quality attributes, control strategy, and risk assumptions). When this level of specificity is missing, CDMOs are forced to make assumptions that may later require costly realignment.
Biotechs that invest time in refining their RFPs—ideally with cross-functional input from analytical, regulatory, and CMC teams—set a more realistic foundation for the work ahead.
Internal Readiness and the Role of the Project Manager
Another common obstacle to efficient execution is the lack of a dedicated internal project manager (PM) with adequate scientific knowledge and decision-making power. Without this role, communication between the biotech and CDMO becomes delayed or scattered, and critical issues are either escalated too late or not addressed consistently.
Sponsors should ensure that the internal PM:
- Has cross-functional visibility (analytical, quality, clinical, regulatory)
- Maintains continuity of communication with the CDMO’s lead team
- Has the authority to approve scope clarifications and contingency decisions
The internal PM is not merely a coordinator but a key enabler of technical alignment and real-time decision-making.
Minimizing Scope Creep through Defined Milestones
Scope creep is a persistent challenge in CDMO–biotech engagements. In many cases, this results not from intentional expansion but from insufficient initial planning or evolving sponsor expectations.
To mitigate this, biotechs should:
- Define clear go/no-go decision points tied to data generation.
- Establish predefined buffers for non-critical change requests.
- Incorporate change control protocols into the governance plan.
Project charters should explicitly list what is in scope, what is excluded, and how changes will be assessed from both technical and budgetary standpoints. CDMOs can only protect timelines if biotechs provide a stable scope of work supported by decision logic.
Building Tech Transfer Maturity
In early-phase collaborations, many biotechs struggle to assemble a complete and coherent technology transfer package. When methods are only partially qualified, or formulation data is preliminary, the burden of translation shifts to the CDMO—introducing risk, rework, and variability.
To improve transfer efficiency, biotech companies should:
- Use standardized tech transfer templates with predefined quality criteria.
- Provide method validation status and known method limitations.
- Include risk assessments with development history and troubleshooting insights.
Even when documentation is incomplete, transparency regarding the current state of knowledge allows CDMOs to plan development workstreams better and avoid duplication of effort.
Communication is a System, not a Meeting.
Finally, communication failure is rarely about frequency—it is about structure. Biotechs often rely on status meetings without a broader operating rhythm, leading to reactive behavior and decision paralysis.
Successful partnerships incorporate:
- Clear communication protocols with named points of contact
- Joint dashboards for real-time tracking of progress, deviations, and risks
- Phase-specific governance models that evolve with programming maturity
Sponsors must also ensure that internal escalation pathways are well-defined—so that urgent decisions do not get bottlenecked by indecision or lack of clarity about authority.
What CDMOs Must Do: Operating with Scientific Authority and Strategic Maturity
While much of the discussion about biotech–CDMO collaboration centers on sponsor readiness, CDMOs themselves must advance beyond just compliance and capacity rhetoric. In complex programs, especially those involving advanced modalities like ADCs, oligonucleotides, and high-potency APIs, scientific expertise alone is insufficient. CDMOs need to operate with strategic maturity—showing not only the ability to follow instructions but also the capacity to guide, anticipate, and co-create solutions in real time.
This section distills the expectations placed on CDMOs by biotech partners and outlines the institutional behaviors, systems, and capabilities required to meet them.
Engage Early with Scientific and Regulatory Perspectives
CDMOs that excel in complex programs consistently provide a technical voice early in discussions—during RFP review, feasibility assessment, and early protocol transfer. This scientific involvement is not just courtesy; it is a risk mitigation strategy.
Biotechs seek CDMO partners who can:
- Identify regulatory gaps or risks based on limited data packages.
- Translate early analytical signals into downstream manufacturing implications.
- Provide formulation or method development input aligned with the molecule’s lifecycle.
Failure to embed this perspective early results in downstream inefficiencies, scope renegotiation, or missed critical development windows.
Build Systems for Transparency and Traceability
In fast-moving collaborations, trust is built through data visibility and process traceability. CDMOs that rely on ad hoc updates, PDF-based communication, or siloed systems cannot meet modern expectations for responsiveness or control.
Leading CDMOs are now implementing:
- Real-time dashboards that provide shared access to batch progress, deviations, and documentation status
- Integrated LIMS and project portals that reduce redundancy and version conflicts
- Standardized tech transfer and method qualification templates accessible to both teams
These systems not only improve delivery fidelity but also create a digital thread that supports audit readiness and regulatory defensibility.
Balance Flexibility with Phase-Appropriate Compliance
Biotechs often face tight deadlines—driven by funding milestones, clinical schedules, or board expectations. CDMOs need to respond to these demands without sacrificing compliance or scientific quality.
The hallmark of a mature CDMO is its ability to say:
“Yes, we can move fast — and here is how we’ll do it without sacrificing control.”
This requires:
- Risk-based quality frameworks that adjust controls based on the development phase
- Predefined “fast-track” pathways that align CMC activities with regulatory strategy
- Scenario planning tools that identify bottlenecks before they occur
In this context, flexibility is not improvisation; it is disciplined agility, supported by structured decision logic.
Invest in Talent That Understands Modality Nuance
Complex modalities demand specialized scientific capabilities, including linker-payload chemistry, oligonucleotide synthesis, and conjugation platform development. More than equipment or SOPs, it is the depth and retention of scientific talent that defines a CDMO’s ability to manage risk, respond to ambiguity, and engage as a technical peer.
Best-in-class CDMOs demonstrate:
- Cross-trained scientists capable of integrating analytical, formulation, and process perspectives
- Structured onboarding and internal knowledge transfer mechanisms
- Visible career development pathways to retain niche talent in high-demand areas
The biotech sector is watching not just what CDMOs build — but who they build with.
Communicate Risk Proactively and Constructively
A repeated point of frustration from biotech leaders is the lack of transparent communication around timeline risks. Delays are often inevitable in development. What separates competent CDMOs from strategic partners is how and when they communicate with them.
Proactive CDMOs:
- Share impact assessments before the sponsor asks
- Escalate based on triggers tied to timeline or quality thresholds.
- Propose mitigation strategies collaboratively rather than defensively.
This approach not only protects trust but also improves decision speed and regulatory positioning.
Lead Lifecycle Thinking from the Outset
Many biotech programs face fragmented planning—where early-phase decisions lead to complications on a commercial scale. CDMOs that adopt a lifecycle-integrated approach help sponsors make better trade-offs early.
This includes:
- Guiding phase-appropriate method validation with scale-up in mind
- Aligning formulation choices with long-term manufacturability and stability
- Advising on regulatory documentation that enables future bridging
CDMO’s role is not merely to deliver units; it is to de-risk development across the whole product lifecycle.
The expectations for CDMOs have changed. Sponsors now want partners who can smoothly integrate into their development ecosystem, provide scientific insights, ensure strict compliance, and advance progress with foresight. Operating at this level demands more than just technical skills; it requires strategic insight, transparency driven by data, and consistent reliability, even under pressure.
In a market driven by scientific ambition and operational constraints, CDMOs that serve as strategic co-developers—rather than just service providers—are the ones that consistently deliver value and build trust.
Shared Mistakes, Shared Lessons: Where Collaboration Breaks Down — and Why
Despite good intentions, CDMO–biotech partnerships often break down—not because of a lack of scientific knowledge or tools, but due to repeated behavioral and strategic mistakes. Input from CDMO executives and biotech leaders shows that common failure patterns exist. These lessons highlight that successful partnerships are rarely ruined by technical issues alone, but by unseen problems in alignment, ownership, and communication.
Misaligned Definitions of Success
One of the most common points of breakdown is the absence of a shared understanding of what success looks like at each stage of the program. While biotechs may prioritize clinical milestone acceleration, CDMOs may interpret “success” as compliance with specifications and audit-readiness. Without explicitly aligned definitions—embedded in governance charters and decision matrices—project teams tend to work at cross purposes.
Success criteria should be co-authored, not assumed. This includes technical endpoints, timeline tolerance thresholds, risk posture, and escalation paths. Alignment at kickoff is insufficient; it must be reinforced at each phase gate.
Scope Creep Without Structured Change Management
Scope creep is inevitable in development programs, particularly as additional assays, formulation modifications, or regulatory clarifications surface mid-project. However, many biotech sponsors expect flexibility from CDMOs without formally renegotiating deliverables, timelines, or budgets. This results in internal CDMO strain, timeline erosion, and eventual confrontation.
Change is not the problem; unstructured change is. CDMO–biotech agreements should include predefined change control frameworks with agreed timelines for re-evaluation, decision triggers, and impact assessments.
Delayed Communication of Risks and Deviations
A critical error—reported frequently by both CDMOs and sponsors—is the tendency to delay difficult conversations. Whether it’s a deviation that might affect a batch disposition, a slipped method transfer, or resource constraints due to concurrent programs, silence or soft communication of risk breeds mistrust.
The threshold for escalation should not be urgent, but potential impact. High-performing partnerships implement project risk registers with shared access and clear ownership for real-time status updates.
Poor Translation Between Functions
Scientific rigor often breaks down at the interface of functions—most notably between analytical and process development, or between regulatory strategy and operations. Many CDMOs and biotechs still operate in siloed functional hierarchies, causing rework, inconsistent documentation, and last-minute regulatory compromises.
Function-specific excellence is necessary, but insufficient. What matters is functional integration, which can be supported through cross-functional working teams, co-authored protocols, and shared accountability for deliverables.
Underestimating Cultural Compatibility
Finally, several panelists emphasized that cultural fit—the willingness to share information openly, the tolerance for ambiguity, the mutual respect for timelines and quality expectations—is a leading indicator of project success. Technical capability is essential, but without shared operating norms, even well-resourced projects can become combative and inefficient.
Sponsors and CDMOs must assess cultural alignment early, not post-contract. This includes expectations on responsiveness, documentation discipline, decision-making speed, and feedback culture. Pilot projects or limited-scope trials can serve as valuable diagnostic tools before expanding the engagement.
The most damaging failures in CDMO–biotech relationships are not dramatic—they are cumulative. They stem from assumptions left unspoken, risks left unflagged, and decisions left ambiguous. These failures are avoidable. When both parties institutionalize clarity, build systems for joint accountability, and create space for early tension detection, collaboration becomes not just functional but scalable.
The Missing Layer: Leadership as Operating Infrastructure
Technical competence and communication frameworks, while essential, are not enough to ensure robust CDMO–biotech collaboration. What often distinguishes successful partnerships is not how well systems are designed, but how leadership behaviors shape decision-making, risk management, and accountability under stress.
In high-complexity programs—particularly those involving novel modalities or accelerated timelines—uncertainty is inevitable. The ability to navigate that uncertainty depends on leadership, not as a function of title, but as an operating system. This includes how teams align, how decisions are made when data is incomplete, and how conflict is resolved without escalation.
These capabilities cannot be outsourced. Nor can they remain siloed. They must be embedded across the interface of sponsor and CDMO teams.
Leadership by Capability, Not Org Chart
Traditional collaboration models assign decision-making power by organizational hierarchy—biotech sponsors lead strategy, CDMOs execute tasks. But complex development demands a more fluid, capability-based approach.
Cross-functional mosaics, not individual experts, lead successful programs. These teams integrate regulatory foresight, analytical depth, quality mindset, operational fluency, and scientific agility into every project phase.
Key leadership capabilities include:
- Strategic translation: ability to connect clinical or regulatory goals with CMC execution
- Technical fluency across functions: particularly at the intersection of process, analytical, and formulation science
- Scenario planning and ambiguity tolerance: to adapt proactively under compressed timelines or shifting scope
- Ownership mindset: regardless of company boundary, functional role, or reporting line
From Meetings to Operating Models
Too often, “collaboration” is reduced to regular meetings and status updates. But meetings do not build alignment—systems do.
Transformative partnerships implement:
- Joint governance councils blending sponsor and CDMO leads across regulatory, analytical, and manufacturing functions.
- Shared accountability structures with co-owned milestones and KPIs
- Transparent escalation paths that empower frontline teams to resolve issues rapidly
- Integrated OKRs (objectives and key results) that drive shared behavior, not parallel effort
This is not about adding layers of oversight, but replacing reactive communication with proactive coordination mechanisms that scale.
Culture Is a Leadership System
Culture—how people behave when decisions are complex—is not the job of HR. It is a reflection of what leaders reward, tolerate, and model.
High-trust collaborations emerge when:
- Teams feel safe surfacing risks before they escalate.
- Leaders model transparency rather than defensiveness
- Constructive dissent is encouraged and integrated into decision-making.
- Feedback loops are fast, non-punitive, and anchored in shared outcomes.
These traits are not aspirational; they are now prerequisites for operating at the speed and complexity required by today’s biotech programs.
AI, Automation, and Digital Fluency as Leadership Leverage
As CDMOs and biotechs expand their digital infrastructure, leadership must evolve to own the application of tools such as LIMS, collaborative portals, predictive risk modeling, and proposal automation—not merely delegate them to IT or operations.
Digital maturity in a collaborative setting requires:
- Understanding how AI can augment forecasting, scenario planning, and stability modeling
- Leveraging dashboards and shared systems for visibility and accountability
- Ensuring that data quality and decision logic remain intact as speed increases
The future of development execution is digital—but only if leadership systems are ready to translate that capability into value.
True collaboration is not built on Gantt charts or signed SOWs. It is built on shared leadership behaviors that drive scientific integration, operational agility, and decision clarity. The absence of this layer—despite technical proficiency—often explains why promising partnerships fail.
Redesigning Collaboration: A Co-Execution Framework
In a development environment defined by speed, complexity, and constrained resources, the traditional CDMO–biotech model—based on service-level agreements and technical deliverables—has reached its limits. What’s needed now is a co-execution framework: an operating model that enables two distinct organizations to function with shared clarity, joint accountability, and adaptive execution.
This section outlines the foundational components of strategic co-execution, translating insights from both sponsor and CDMO perspectives into practical structures that enhance scientific integration, operational discipline, and long-term scalability.
Joint Operating Rhythm
A hallmark of effective collaboration is not how often teams meet, but how consistently they act across functions and phases. Co-execution requires a structured operating rhythm that aligns both companies’ activities without redundancy.
Core elements include:
- Phase-specific governance models (e.g., discovery, pre-IND, post-Phase I)
- Shared kickoff frameworks including roles, risk maps, and escalation logic
- Integrated calendar of activities tied to joint milestones—not just internal deadlines
This structure reduces project drift and prevents late-stage surprises.
Co-Owned Technical Risk Register
Most development programs will encounter scientific ambiguity, shifting priorities, or unexpected assay behavior. What differentiates high-performing teams is the ability to identify, document, and manage risk collaboratively.
A shared risk register should:
- Capture scientific, regulatory, and operational risks in a standard format.
- Assign joint ownership and mitigation actions.
- Serve as a live document reviewed in governance meetings.
This approach normalizes complexity and moves teams from reaction to preparedness.
Bi-Directional KPIs and Incentive Alignment
Conventional CDMO performance metrics (e.g., on-time delivery, batch success rates) are necessary but insufficient. Sponsors also need insight into how work is executed—especially when timelines slip or scope evolves. Co-execution introduces bi-directional metrics, such as:
- Sponsor responsiveness and scope stability
- CDMO deviation resolution speed and communication clarity
- Joint adherence to change control timelines
- Shared success indicators (e.g., IND filing quality, audit readiness)
Including these metrics in QBRs and performance dashboards increases transparency and trust.
Digital Integration and Shared Systems
Co-execution is enabled by real-time, secure access to critical data. Many delays stem not from technical issues, but from fragmented systems and asynchronous communication.
Modern partnerships now employ:
- Shared dashboards (timeline, batch progression, document status)
- Collaborative document portals with version control
- Integrated LIMS access or tailored data exports for cross-validation
These tools reduce time lost to clarification, duplicate requests, and information silos.
Scenario Planning and Pre-Approved Contingencies
One of the most effective tools in complex program management is structured scenario planning. Teams that anticipate potential roadblocks—regulatory shifts, method delays, manufacturing constraints—are better equipped to respond without disruption.
Sponsors and CDMOs should jointly define:
- What-if scenarios across timeline, regulatory, and CMC variables
- Pre-approved contingency pathways (e.g., alternative assay validation strategies)
- Financial and timeline impacts for each scenario
This minimizes reactive decision-making and maintains project momentum.
Behavioral Expectations and Decision Logic
The final layer of co-execution is behavioral. Teams must agree on how decisions are made under ambiguity, especially when speed, quality, and cost collide.
Best practices include:
- Documented decision-making frameworks for scientific ambiguity or deviations
- Clarified roles for technical, regulatory, and quality sign-offs
- Expectations around escalation etiquette, issue framing, and feedback cycles
These norms prevent internal friction and enable productive disagreement—a critical trait in innovation-intensive environments.
Strategic co-execution is not a theory. It is an evolving framework that builds resilience, clarity, and speed into CDMO–biotech partnerships. By aligning technical governance, risk transparency, communication infrastructure, and shared behavioral norms, organizations can collaborate more like integrated teams than independent contractors.
Closing: The Partnership Litmus Test
In an industry where timelines are non-negotiable, innovation is high-risk, and regulatory scrutiny is intensifying, the difference between a successful drug development program and a failed one often comes down to how well two organizations work together under pressure.
What has become evident through this panel—and through two decades of hands-on development leadership—is that traditional sponsor–CDMO relationships, based on compliance, contract terms, and tactical coordination, are no longer sufficient. The future belongs to partnerships grounded in strategic co-execution: joint decision-making, shared behavioral norms, and integrated risk ownership. This shift is not just philosophical. It is operational, measurable, and increasingly essential.
The Litmus Test for Modern Collaboration
As both biotechs and CDMOs reflect on their current partnerships, a simple but revealing test can help evaluate maturity:
- When ambiguity arises, do both teams seek clarity—or seek cover?
- When timelines slip, do both sides adjust together—or escalate blame?
- When decisions are urgent, do both parties act with aligned judgment—or retreat into functional silos?
- And when success is achieved, is it viewed as mutual or one-sided?
If the answers to these questions expose hesitation, silos, or reactive behavior, then the partnership is not yet operating at its full potential.
The Real Differentiator Is Not Infrastructure — It’s Behavior
Many CDMOs today boast advanced instrumentation, expanded cleanroom space, and cross-site scalability. Likewise, many biotech firms bring cutting-edge science and promising therapeutic platforms. Yet execution still fails—not for lack of capacity or intellect, but for lack of behavioral alignment under stress.
What defines a high-performing collaboration is not the absence of problems, but the presence of:
- Clarity when priorities collide
- Transparency when risk emerges
- Shared logic when the data is incomplete
- And trust when speed and quality must coexist.
These are the traits that drive not only faster filings and more robust submissions—but also repeat business, team retention, and long-term credibility with regulators and investors alike.
Final Reflection
As complexity deepens and expectations rise, both CDMOs and biotechs face a shared imperative: to rethink how they partner — not just to deliver, but to learn, adapt, and succeed together.
Throughout this panel, leading biotech, CDMO executives shared candid insights from the frontlines — where ambitious science meets operational reality. What emerged wasn’t a single “right” model, but a recurring theme: alignment is everything.
The organizations that will define the next era of drug development won’t be those with the largest footprint or the fastest timelines. They’ll be the ones that master the art of thinking together, deciding together, and moving forward together — anchored in scientific discipline, operational clarity, and behavioral trust.
Because in today’s environment, execution is no longer the finish line.
It is the differentiator. Alignment is the new infrastructure.
LIST OF PANELISTS
Marie-Sophie Quittet
Head of Customer Relationships at
Adragos Pharma, Jura
Alastair Hay1 , Jonathan Loughrey2
1. VP Peptides, Almac Sciences
2. Director of Chemical Development for
Early Phase, Almac Sciences
Matthias Henz, PhD
Partner at Alpha Lyncis AG
Diego Schmidhalter
Partner and Director at Alpha Lyncis AG
Dr. Chandrakanth Gadipelly
Principal Research Scientist & Co-Founder – Amar Flow Laboratory
Amar Equipment Pvt. Ltd. & Amar Flow Laboratory LLP
Subas Sakya
Chief Scientific Officer, BioDuro
Sylvia Wojczewski, PhD
CEO, BioSpring GmbH
Andrew Mitchell
Associate VP, Business Development, BIOVECTRA
Kathryn L. Ackley, PhD.
Oligonucleotide CMC Consultant, USA
Dr. Stephen Houldsworth
Sr. VP, Global Head of Small Molecules Platform, CordenPharma
Dr. Brett Wagner
R&D Search and Evaluation Manager,
Douglas CDMO
Xavier Pujol
CSO & CDMO Business Development
North America, Farmhispania Group
Kenneth N. Drew, Ph.D.
VP Flamma USA, Flamma
Kyriakos Kansos George Ntortas
CDMO Consultants, Fuliginous Management
Consulting (FMC)
Nicholas Shackley
CEO, Gannet BioChem
Robert Hughes
Research Fellow, Grace
Kit Hale, Ph.D.
Founder & Principal, Hale CMC Solutions
Eduardo Paredes, Ph.D., M.B.A.
SVP of CMC, Leal Therapeutics, Inc.
Abdel Aziz Toumi
CEO, Lupin Manufacturing Solutions
Saharsh Davuluri
Vice Chairman & Managing Director,
Neuland Laboratories Limited
Celine Chen1 , Dongxin Zhang2
1. Head of European Business, PharmaBlock
2. Senior Director, Tides Center of Excellence, PharmaBlock
Hodaka Shiraishi
Sales & Project Manager, Procos
Fabien Bonhoure
Global Business Project Director, SEQENS
Jennifer DePolo
General Manager and Site Head of the
Siegfried Acceleration Hub
Joachin Fries
Global Head Project Management Drug Products, Siegfried
Mat Minardi
EVP of Global Project Management,
Sterling Pharma Solutions
Rohtash Kumar
Senior Vice President, Chief Technology Officer, Veranova, L.P.

Marie-Sophie Quittet
Head of Customer Relationships at Adragos Pharma, Jura
What should biotechs include in their initial RFPs to better align CDMO capabilities with project expectations?
A clear and detailed RFP is foundational to a successful collaboration. Sometimes, RFPs are vague or incomplete, leading to misaligned expectations or time-consuming clarifications later on. For an RFP to be effective in the area of fill-finish technologies, biotechs should include the following:
- Project scope and objectives: Clearly define both immediate and long-term goals (e.g., Phase 1 clinical supply versus commercial readiness).
- Timeline requirements: Outline all deadlines, including flexibility windows and any critical milestones for funding or clinical trials.
- Technical and regulatory requirements: List product characteristics (e.g., biologic modality, expected batch sizes, necessary technologies such as lyophilization or single-use systems, desired sterilisation method, security information such as cleaning and safety data sheets (SDS), regional regulatory constraints, and the preferred quality standards (e.g. FDA, EMA, JP).
- Material specifications: Share as much as possible about the API, excipients, packaging preferences, and whether ready-to-use components are preferred.
- Analytical methods and expectations: Be explicit about the analytical support needed, stability study design, and expectations for method development or transfer.
- Logistics and supply chain needs: Note any special requirements for storage, shipping, and material tracking.
Importantly, I would recommend sharing any uncertainties openly. For instance, whether certain analytical methods might not yet be fully validated, or if clinical timelines are dependent on funding. This transparency enables the CDMO to propose the best options and flag any feasibility or risk factors early on.
What steps can biotechs take to define project requirements better and avoid unnecessary delays?
Project delays in biotech-CDMO collaborations often stem from incomplete upfront alignment on what is required and when. Biotechs can save considerable time (and resources) by investing in thorough preparation before engaging with a CDMO. Key actions include:
- Internal alignment: Ensure all stakeholders (regulatory, clinical, supply chain) agree on the project’s objectives and constraints prior to engaging the CDMO. This reduces change orders and confusion later.
- Feasibility assessment: Where possible, conduct a pre-feasibility review (sometimes with input from your chosen CDMO) to identify technical gaps (e.g., need for filter validation, specific leachables studies) and regulatory issues.
- Prioritize requirements: Distinguish between essential and “nice-to-have” deliverables, particularly for early phase projects where resource efficiency is crucial.
- Define analytical requirements early: Many delays arise when analytical methods are unavailable or unsuitable for intended IPC (in-process controls) or release specifications; engage QC teams early and be explicit about what, when, and where analytics are needed.
- Prepare for material supply and readiness: Given current global supply chain risks, clarify with the CDMO lead times for critical materials (e.g., filters, excipients), and consider agreeing on safety stocks or backup suppliers.
At Adragos Jura, we have often accelerated timelines for clients who provide detailed material readiness plans, and proactively address potential gaps, such as defining appropriate volumes to discard post-filtration or aligning on buffer preparation methods in advance.
How can biotechs establish more apparent communication channels to avoid misalignment with CDMO teams?
Open, structured communication is the backbone of any successful biotech-CDMO partnership. Misalignment frequently occurs when communication is either too infrequent or routed through too many, or too few, channels. To mitigate this, I would recommend the following:
- Single point of contact: Nominate a dedicated project manager on the biotech side (ideally mirrored by a CDMO project lead). This streamlines decision-making and accountability.
- Kick-off meetings: Hold formal project kick-off sessions with all stakeholders present, including those from analytics, QA, production, logistics, and regulatory teams, to ensure a shared understanding of objectives, timelines, and critical process steps.
- Regular progress updates: Implement weekly or bi-weekly project calls, with agendas and action items circulated in advance. For complex or multi-site projects, shared digital project trackers (e.g., Gantt charts or dashboards) are invaluable.
- Clear documentation and traceability: Ensure that manufacturing and analytical records are available in real time to both parties, fostering transparency and enabling rapid response to unforeseen issues.
- Escalation pathways: Predefine how and when issues will be escalated, both for urgent operational matters and for strategic scope or regulatory questions.
Our most successful biotech clients at Adragos Jura are those who treat the partnership as a joint venture, not a one-way service, actively participating in regular reviews and openly flagging upcoming challenges or changes.
What advice would you give biotechs about balancing cost, quality, and timelines when selecting a CDMO?
Balancing the “iron triangle” of cost, quality, and speed is one of the critical strategic tasks for any biotech team engaging a CDMO, and it inevitably requires tailored trade-offs at different stages of development:
- Early phases (clinical supply): Prioritise flexibility and scalability over the lowest cost per vial. A CDMO with adaptable equipment and experienced aseptic staff may save months in tech transfer and troubleshooting later, even if unit costs appear higher.
- Later phases (commercial readiness): Quality and regulatory robustness come into sharper focus, as does the reliability of supply. Here, proven audits, robust QA teams, and global regulatory expertise (e.g., FDA, EMA, PMDA experience) become non-negotiable.
- Cost transparency: Insist on clear, detailed proposals from the CDMO that identify all deliverables, pricing models, and the nature of out-of-scope work. Avoid bundled pricing where possible; options à la carte encourage transparency and trust.
- Mutual risk management: Understand what risks (timeline, technical, supply chain) each party is bearing and clarify upfront how these are managed and mitigated. For critical activities, such as custom materials or unique sterilisation steps, ensure that “what-ifs” are discussed and costed.
- Invest in partnership: Both sides should be prepared for flexibility. At Adragos Jura, we accommodate accelerated schedules or documentation customisations for clients if needed, who are willing to share clear project priorities and mutual risks; this approach often results in both cost and time savings by focusing resources strategically.
In summary, a high-trust, collaborative relationship anchored on transparent communication and mutual understanding of risks and deliverables is far more likely to yield both successful clinical outcomes and sustainable partnership value.

Alastair Hay
VP Peptides, Almac Sciences

Jonathan Loughrey
Director of Chemical Development for Early Phase, Almac Sciences
How do you approach early-stage discussions with biotechs during RFP or feasibility phases?
Initial engagement depends on the detail provided ranging from brief emails to full technical packages. Clarifications are common, and a call is usually the best way to align scope. For complex projects, face-to-face meetings are ideal to ensure mutual understanding and accurate proposals.
What scientific support can CDMOs or CROs provide to biotechs during lead optimization and candidate selection?
Support is either transactional (e.g. FTE-based synthesis and analysis) or enhanced with specialised services which Almac Sciences offer, e.g. polymorph screening, solubility/stability profiling, and counterion screening for peptides. These help biotechs assess and refine candidate molecule effectively.
At what stage in drug discovery do you recommend CDMO involvement to ensure smoother downstream development?
The obvious answer is to say “as soon as possible”, but that doesn’t always suit the biotech’s model, and the CDMO must always show a degree of flexibility. It is important that the biotech knows their molecule and that may be best served by having internal resource with hands on experience of synthesising and using it. If all the biotech’s experience comes from outsourced data, then it can make it more difficult to resolve technical challenges.
How do you align your technical team’s input when biotechs bring discovery-stage molecules with limited characterization or formulation data?
Limited data is common. Fortunately, at Almac Sciences, we have tremendous breadth and depth of capabilities, and these can be tailored to the details of the technical package at quotation stage with recommendations for project scope. Building a characterisation package (e.g., pKa, pI, log P/D) is recommended as it helps guide formulation strategies aligned with the intended administration route.
What capabilities have you built to support the transition from hit-to-lead or preclinical discovery to IND-enabling studies?
Rapid synthesis and design input are key and since this phase of work is often investigative in nature, it is typically best supported by FTE programmes. Early formulation scientists can also use characterisation data to engineer properties like solubility and stability, adjusting buffers, excipients, and surfactants to meet dosing requirements.
What best practices help ensure scientific alignment between CDMO/CRO and biotech analytical, formulation, and process development teams?
Strong project management is essential. Almac Sciences fosters technical-technical communication between its team and sponsors. Project managers and technical leads collaborate closely to maintain alignment and ensure smooth execution.
How do you plan internal resources and capacity when handling multiple biotech clients in parallel without compromising timelines or quality?
A project manager is assigned after contract signature. Departmental managers allocate resources based on portfolio needs, with regular reviews to identify availability and pinch points. Almac Sciences’ strong interdepartmental relationships support efficient delivery.
How do you communicate timeline risks and mitigation strategies effectively to biotech partners?
Almac Sciences emphasises scheduling from project kick-off, identifying critical path activities and milestones. Risks are communicated early, including sponsor-side bottlenecks like document reviews. Transparency via regular update meetings ensures shared responsibility and proactive mitigation.
What practices do you follow to ensure transparency, accountability, and traceability in complex development programs?
Communication is key, and Almac Sciences’ culture is very much one of information sharing from both a technical and project delivery perspective. Regular project meetings, clear notes with agreed decisions and tracked actions are crucial to ensuring successful project delivery.
How do you support complex modality onboarding (e.g., oligonucleotides, ADCs, PROTACs) from early-phase biotech companies?
Onboarding what may be viewed as a complex modality is no different from what may be perceived to be a simpler one, ie a small molecule. An expert provider is exactly that – they have the technical and regulatory know-how and the internal capabilities – people, equipment, analytical instrumentation – to be able to deliver the project. New modalities often have specific considerations to consider that may not match small molecule experience, and care has to be taken that a fit for purpose approach is adopted.
What tools or digital platforms (e.g., dashboards, LIMS, collaborative portals) have improved real-time visibility and engagement with biotech clients?
Digitisation enhances collaboration. Client-specific portals streamline file sharing and document review. Electronic approval tools replace traditional methods, improving efficiency and visibility throughout the project lifecycle.
What’s your approach to method development and tech transfer when receiving incomplete or early-phase packages from biotechs?
Thorough technical assessment of the RFP ensures accurate proposals. Incomplete data will likely increase the required development time, but it’s not unusual that information will be partial, or there is guidance from the client that improvement is required. The key is communication and strong technical assessment so that we minimise the chance of surprises – no one likes those!
Some modalities lend themselves to general rather than product or process specific knowledge. For example, in peptide projects, it is uncommon for high levels of detail to be provided on a manufacturing process at the preclinical stage. Instead, general expertise and practises are applied and amended on a product-by-product basis.
How do you support “fast-to-clinic” timelines while maintaining regulatory compliance and phase-appropriate quality?
This is a very interesting area and one Almac Sciences has paid a lot of attention to. Personalised medicine, e.g. cancer vaccines, demands speed and tailored regulatory expertise as each product is “made to order”. GMP principles remain, but streamlined processes are applied following appropriate justification. For conventional drugs, phase-appropriate development and integrated services accelerate timelines.
How do you attract, train, and retain specialized scientific talent in high-complexity areas such as linker-payload chemistry, RNA synthesis, and high-potency API handling?
Operating in a niche modality would imply a smaller talent pool, but often these specialised areas can be sold on the basis of their uniqueness and exciting prospects. Recruitment should focus on core skills over narrow experience; internal training is expected, and flexibility in hiring ensures access to capable scientists who can grow into specialised roles.
What examples or lessons learned can you share from both successful and challenging biotech partnerships?
Empathy and collaboration drive success. Progressing a molecule from the bench to the clinic is an extremely difficult thing to do, and challenges will arise first time round. Strong relationships help navigate first-time challenges. Transactional partnerships struggle with problem resolution and understanding each other’s pressures fosters resilience and solution-oriented teamwork.
How do you assess and communicate your ability to support lifecycle development—from early-phase through to commercialization?
Almac Sciences has experience of taking products from pre-clinical right the way through to commercial, and others that transfer in at a later stage. The same fundamentals apply across the spectrum of projects – regulatory adherence is critical. The real expertise lies in navigating critical considerations, such as determining the starting point for Process Validation and shaping the GMP-compliant manufacturing strategy accordingly. Practical aspects, like ensuring compatibility with the required production scale, also play a significant role in successful progression.
What qualities or behaviours define a successful biotech partner from your perspective?
In my view, the most effective relationships form where the sponsor is engaged but not overbearing. Too close, and the CDMO will be smothered, too distant, and there is insufficient grasp of the true project status. The ideal sponsor possesses enough technical and regulatory knowledge to challenge and understand CDMO work. Mutual empathy and respect ensure productive collaboration and effective problem-solving.

Matthias Henz, PhD
Partner at Alpha Lyncis AG
Evaluation of CDMOs
During initial due diligence, how do you evaluate a CDMO’s quality management system?
The article is a concise, forward-looking blueprint for QMS appraisal forcing due diligence teams to inspect how quality is embedded, not just whether documents exist.
The author proposes a three-pillar framework to guide the evaluation of a quality-management system (QMS) at a CDMO:
First, regulatory compliance is verified through classic audit techniques, ensuring that all essential procedures exist and satisfy the minimum GMP requirements.
Second, the confirmation of a modern QMS design is done by looking for a modular procedure architecture, verifying the existence of lifecycle-aligned control strategies that grow from R&D to commercial supply and of built-in risk-based standardized approaches. Furthermore, procedures are supposed to be lean containing a series of strong visualization elements so that an independent reader is capable to understand the content of a procedure within five minutes – the “IKEA principle”.
Third, the depth of the embedded GMP culture and leadership governance is assessed by examining clear escalation pathways for significant events, data-driven KPI dashboards with documented follow-up actions, and management-review processes (Quality Council) that lead to tangible improvements.
The author believes that a CDMO demonstrating strength across all three pillars provides sponsors an enhanced assurance of sustained product quality and patient safety.
Traditional Due-Diligence Approach
In most CDMO due-diligence reviews, sponsors concentrate on the documented components of the Quality Management System (QMS) and on evidence of compliance adherence to procedures. Although essential, these document-centric review and compliance assessment provide a static snapshot and rarely illuminate whether the system functions dynamically enough to foster a mature quality culture. A robust due diligence program should therefore also scrutinize the QMS architecture and ascertain the depth of senior management’s day-to-day engagement in quality activities.
Modular QMS Architecture
During a due-diligence review, sponsors should verify that the CDMO’s Quality Management System (QMS) is organized in modular architecture – discrete yet inter-linked policies and procedures that govern each functional domain. This structure delivers operational flexibility and procedural clarity, allowing individual SOP to be introduced, revised, or retired without disrupting the entire system. Consequently, improvements can be prioritized according to organizational maturity or emerging regulatory requirements, keeping the QMS agile and aligned with sponsor expectations.
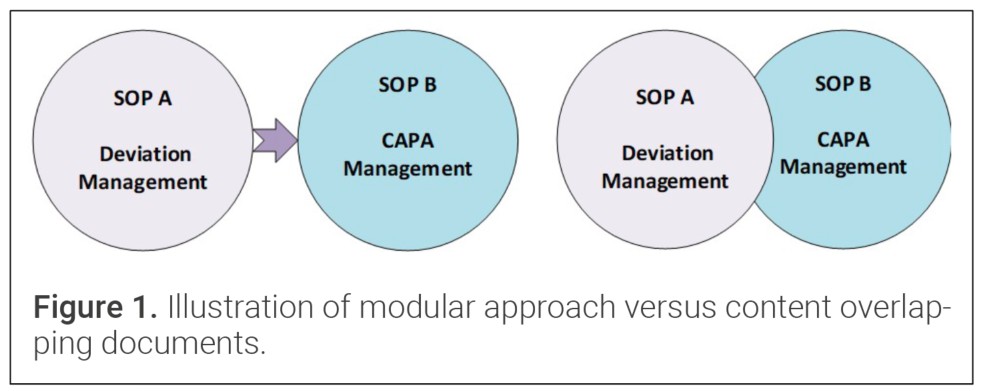
Figure 1 illustrates why a modular design must also enforce clear boundaries between documents. The left panel depicts the desired state: two procedures with distinct, non-overlapping content. The right panel shows redundant content appearing in both procedures, which increases complexity and invites inconsistencies during future revisions – a risk that should be eliminated in any CDMO QMS. By defining precise remits and directing users to other procedures, rather than replicating related content, a modular framework safeguards against such content duplication.
Lifecycle-Driven, Risk-Embedded QMS Design
Modern QMS architecture should incorporate a lifecycle-based procedural framework that reflects GMP, compliance, and regulatory expectations in line with product maturity – from API starting materials (API-SM) and GMP intermediates to full-scale commercial APIs. Aligning procedures with each clinical and commercial phase guarantees compliance with ICH Q7 (including Chapter 19) and other prevailing standards (refer to Figure 2).
Within this framework, a CDMO must also anticipate the inherent, foreseeable risks that accompany its systems and processes. Whenever such risks affect quality, compliance, or operational integrity, control measures should be built directly into the relevant procedures. Embedding these “risk-designed” controls at the outset ensures that predictable hazards are managed systematically, prevents routine issues from escalating into deviations, and delivers consistent risk mitigation throughout the manufacturing lifecycle.

Visual-First QMS Design: The Five-Minute Rule
A contemporary CDMO QMS should adopt a visual-first philosophy that accelerates comprehension, simplifies navigation, and enhances transparency. Under this model, any user – regardless of function or seniority – should be able to grasp the purpose, scope, and structure of a procedure within five minutes (“the Five-Minute Rule”).
To achieve this, policies and procedures are augmented with purpose-built visual aids:

This approach mirrors the picture-led instructions “IKEA Principle”: complex furniture assemblies are distilled into clear visuals that let untrained individuals build reliably the first time right. Translating that principle to pharmaceutical operations replaces text-heavy procedures with visualization aids, markedly avoiding misinterpretation and deviation while sustaining full GMP compliance and reducing cost.
Management-Engagement for Proactive Quality Governance
During due diligence, the sponsor should not only verify the existence of governance procedures but also how effectively they are embedded in daily practice. A robust CDMO QMS therefore includes dedicated Management-Engagement related procedures that make senior leadership directly accountable for quality performance, risk-based decision-making, and continuous improvement. Key elements include:

These procedures and their application empower management to track performance in real time, address emerging issues before they escalate, and ensure that quality objectives remain tightly coupled to corporate goals – hallmarks of a mature QMS at a CDMO, which fosters confidence at the sponsor.

Diego Schmidhalter
Partner and Director at Alpha Lyncis AG
Technology Transfer - More than Handing over Paper
How do you assess and communicate your ability to support lifecycle development from early-phase through to commercialization?
All partners of Alpha Lyncis have more than 25 years of experience, mostly in the CDMO industry, in various senior operations and business management functions in R&D, MSAT, manufacturing operations, quality, technical innovation and business development. This enables our experts to provide broad strategic and technical support over a majority of relevant business areas and business processes and over a broad range of production technologies such as for microbial, cell-culture based products, chemical synthesis of APIs, peptides and oligonucleotides, ADC, mRNA, biotransformation, biocatalysis and others.
Our customer base includes start-ups, SMEs and large pharma companies, including CDMOs. The focus of our service is on enabling our customers to be more effective and efficient, by identification and implementation of best-in-class process solutions, accelerating process development and de-risking programs and technology transfers through early identification of pitfalls and risks. Other focal points include the development and implementation of a holistic strategy for single-use technology, definition of equipment lay-out, the modular implementation of a turnkey quality management system and the use of our extensive professional network to bring together project partners.
At what stage in drug discovery do you recommend CDMO involvement to ensure smoother process development and what services can a CDMO provide?
Focus of drug discovery is on identifying a product, showing superior performance in a treatment or in the prevention of a disease. CDMOs typically enter the stage when it comes to develop a production process, suitable for future commercial manufacture which, in the case of cell-based production, comprises strain or cell-line development.
The choice of a best production organism is paramount. This is where experience of a CDMO comes into play. Leading CDMOs offer access to superior expression technology in combination with high-throughput (HTP) screening and HTP process development capabilities. This combination allows to significantly speed up the development of clones, selection of a best producing clone, fermentation or cell culture and purification development. Unlike most biotech companies, CDMOs work on a large number of products within a one-year period, which significantly adds to the expertise of CDMO scientists allowing them to develop high-titer USP processes and high-yield DSP processes while delivering a product that meets specifications.
mRNA products are typically produced using an in-house developed platform process. Therefore, mRNA companies are approaching CDMOs mainly for the production of pDNA or for the manufacture of product i.e., production of mRNA bulk substance and final formulated bulk drug.
Due to the high risk involved, biotech companies are advised to choose a CDMO partner very carefully as cost for developing a product are considerable and high regulatory standards must be met. Entering into a collaboration is usually the start of a long-term relationship, demanding a good cultural fit. A defined list of key selection criteria, previous experience and knowledge of the CDMO sector at a global level, including an understanding of the associated costs, are important prerequisites for selecting the best possible partner.
What best practices help ensure scientific alignment between CDMO/CRO and biotech analytical, formulation, and process development teams?
There are numerous practices that have a positive impact on the alignment between the project sponsor’s team and the service provider’s team. To mention just a few:
- Open communication and mutual trust are key. It begins as early as the RFP-Proposals phase. Already in this phase the two teams should be connected to ask and answer question to develop a mutual understanding of the current status of process and analytical development and the properties of the product. A thorough assessment helps to determine the starting point and to verify the deliverables.
- A detailed project plan based on standardized work-flows should list project deliverables and timelines.
- A clear project team structure, led by program managers on both sides, supports smooth interaction between the teams as well as having an escalation process in place. This protects scientists from being drawn into stressful discussions they have no decision-making authority.
- Weekly cross-team meetings, to share results and discuss observations.
- Process development scientists and an analytics expert of the sponsor should be present in the CDMO’s laboratories during the technology transfer, i.e. to support the first verification batch(es).
- Technology transfer should base on a structured approach. Overall, we made good experience with following a two-level stage gate process, a first thorough assessment of the project development status after finalization of the initial verification batches to address potential changes to the program and a second stage gate after completion of the agreed program to assess production readiness. Examples of aspects to asses during the stage gate reviews are: robustness and reproducibility of each unit operation, product stability, availability of analytical methods, raw materials and biosafety, EMC, safety challenges, equipment suitability and cleanability and process economy.
- I also advice the use of visual standard work plans to reduce friction in a complex working environment, such as tasks requiring collaboration of multiple departments, campaign preparation being an example.
What tools, digital platforms have improved real-time visibility and engagement with biotech clients?
Visual as well as digital tools support communication on different levels between the sponsor company and the CDMO. Scientists and program managers focus on adherence to the detailed project plan and progress against that plan. Upper management wants to understand the overall status of a project, with issues and risks highlighted. This can be perfectly visualized in a one-page project dash-board. Some CDMOs are sharing project-specific real-time data by giving access to their global data mart or LIMS.
What lesson learned can you share from both successful and challenging biotech partnerships?
The relationship between sponsor and CDMO is a collaborative effort based on trust. Therefore, maintaining a good relationship between the involved is of crucial importance. This includes maintaining a positive attitude, assuming that both parties are striving for the best possible project success. Trust cannot be taken for granted. So, it is important to immediately communicate issues and incidences, however unpleasant the message may be, whether the issue is due to force majeure or improper action – a rule, that is often not followed.

Dr. Chandrakanth Gadipelly
Principal Research Scientist & Co-Founder – Amar Flow Laboratory
Amar Equipment Pvt. Ltd. &
Amar Flow Laboratory LLP
Biotech-CDMO Collaboration: A 2025 Perspective of a FlowChemistry Veteran
By 2025, the biopharmaceutical industry will showcase unprecedented diversity, including antibody-drug conjugates (ADCs), therapeutic oligonucleotides, mRNA vaccines, gene-editing payloads, and next-generation peptide conjugates. This expansion is invigorating innovation but simultaneously intensifying competition for specialized Contract Development and Manufacturing Organization (CDMO) capacities. Challenges compounded by fragile global supply chains, stringent regulatory scrutiny, and investor-driven urgency for rapid “idea-to-IND” timelines have transformed traditional outsourcing into deeply integrated strategic partnerships.
Key Structural Shifts Influencing Collaboration
Three pivotal structural shifts have emerged:
- Convergence of High-Hazard Modalities: New therapeutic modalities often involve potent toxins, hazardous chemical linkers, and complex synthetic intermediates. For example, ADCs incorporate high-potency payloads; siRNAs use fluorinated phosphoramidites; CRISPR therapies require precise synthetic controls. Consequently, advanced containment systems and highly automated micro- and meso-flow reactor technologies are imperative to safely handle these substances.
- Accelerated Regulatory and Digital Expectations: The formalization of continuous manufacturing through regulatory frameworks like ICH Q13 and initiatives such as the FDA›s Framework for Advanced Manufacturing (FAM) have significantly increased demands for stringent data integrity and real-time batch release capabilities. Sponsors now anticipate CDMOs to provide transparent, compliant electronic batch records with real-time monitoring.
- Geopolitical and ESG Pressures: Government-led reshoring incentives in the US and EU, alongside India’s Production-Linked Incentive (PLI) schemes, are reshaping global capacity distribution. Moreover, Environmental, Social, and Governance (ESG) criteria, particularly Scope-3 carbon emissions reporting, prioritize solvent minimization, single-use flow equipment, and renewable energy sources.
Navigating this landscape demands meticulous partner evaluation, realistic scheduling, disciplined governance, and transparent communication between biotechs and CDMOs from the outset.
Critical CDMO Evaluation Criteria in 2025
To select suitable CDMO partners, biotechs should prioritize:
- Modality-Specific Technological Depth: Ideal CDMOs demonstrate robust continuous-flow capabilities for high-potency active pharmaceutical ingredients (HPAPI) validated at over 10 kg/year scales, equipped with inline analytical monitoring systems like FTIR and UHPLC. Specialized purification techniques such as tangential-flow filtration, dual-column chromatography for ADC payloads, and enzymatic capping modules for oligonucleotide synthesis are essential benchmarks of advanced technological depth.
- Regulatory and Quality Maturity: Top-tier CDMOs exhibit impeccable regulatory records, demonstrated by zero repeat observations in recent FDA or EMA inspections, robust electronic Quality Management Systems (e-QMS) compliant with ALCOA-plus principles, and active participation in regulatory innovation programs such as FDA›s FAM pilot.
- Operational Flexibility and Buffer Capacity: Facilities should feature modular cleanroom setups capable of rapidly transitioning between product modalities (e.g., cytotoxic compounds to oligonucleotides) within short turnaround times, supported by well-trained, multidisciplinary teams. Stable staffing with turnover rates below 10% ensures consistent quality and continuity.
Validating CDMO Performance and Fit
Biotechs can validate CDMO capabilities through:
- Reviewing multiple randomly-selected project schedules (Gantt charts) for timeline adherence, noting cumulative delays as indicators of reliability.
- Confirming Right-First-Time (RFT) batch success rates above 90%, where lower scores indicate potential quality or regulatory issues.
Inspecting real-time Process Analytical Technology (PAT) implementations and Statistical Process Control (SPC) charts, as live data streams demonstrate genuine process control versus static, potentially misleading snapshots. - Conducting thorough quality audits by tracking deviations through Corrective and Preventive Action (CAPA) processes, verifying floor-level implementation within defined timelines (ideally under 30 days).
- Assessing regulatory compliance histories, interpreting isolated versus recurring compliance issues, and reviewing active regulatory engagement such as ongoing agency interactions.
- Evaluating infrastructure alignment, ensuring redundancy in utilities (e.g., chillers, air systems), appropriate containment for hazardous materials, and correctly scaled, validated equipment for intended production volumes.
Additionally, CDMOs provide critical scientific support during the lead optimization phase by aiding in identifying scalable synthesis routes, robust analytical method development, and stability evaluations, thus significantly mitigating downstream scale-up risks.
Ensuring Timely Execution
Robust scheduling requires detailed bottom-up planning based on historical data, clear identification of critical paths, proactive incorporation of realistic raw-material lead times (e.g., 20 weeks for phosphoramidite procurement), and contingency planning, including dual-sourcing strategies for critical inputs. Transparent and proactive communication regarding potential risks and implementation of Joint Steering Committees (JSCs) facilitate quick resolution and alignment.
Key performance indicators (KPIs) such as schedule adherence (>90%), deviation closure timelines (<30 days), and Batch Record accuracy (>95%) offer predictive insights into potential project risks. A disciplined project management approach, including clearly defined hold-points and liquidated damage clauses for delays, ensures balanced speed and compliance.
Practical Recommendations for Biotechs
- Construct detailed Requests for Proposals (RFPs) clearly outlining Quality Target Product Profiles (QTPP), anticipated production scales, known hazards, and intellectual property positions.
- Pre-contractually define Critical Process Parameters (CPPs) to control scope effectively and manage change requests via formal documentation systems.
- Establish centralized digital communication platforms for real-time project updates, including visual status indicators and regular briefing sessions.
- Conduct rigorous technology transfer preparations, providing comprehensive documentation and performing gap assessments well in advance of transfer initiation.
- Engage dedicated internal project managers versed in chemistry, regulatory compliance, and finance to effectively oversee CDMO collaborations.
- Pilot small-scale feasibility studies to assess technical capability alignment, communication effectiveness, and cultural compatibility prior to full-scale engagements.
- Use balanced decision-making matrices, weighted towards quality (40%), timeline reliability (35%), and cost considerations (25%), rather than defaulting solely to lowest-cost options.
Conclusion
While complex therapeutic modalities represent substantial clinical opportunities, they simultaneously elevate manufacturing and regulatory risks. Effective collaboration between biotechs and CDMOs is thus crucial. Front-end diligence—thoroughly vetting partner capabilities, ensuring real-time analytical proficiency, assessing regulatory maturity, and fostering transparent communication—represents the single most critical investment in securing timely, successful drug development. Meticulous evaluation and careful planning mitigate risks, ensuring robust operational performance and avoiding costly delays. In 2025’s fiercely competitive biopharmaceutical landscape, such comprehensive due diligence and transparent collaboration are not just advisable; they are imperative for sustained commercial and clinical success.

Subas Sakya
Chief Scientific Officer, BioDuro
How do you approach early-stage discussions with biotechs during RFP or feasibility phases?
At BioDuro, early-stage engagement typically begins with assigning a project manager upon receipt of an RFP. This person coordinates internal efforts to collect detailed responses from the relevant scientific and operational teams. The compiled information is reviewed, validated, and shared with the biotech partner. These discussions often continue as needed to clarify scope, address technical considerations, and ensure alignment on feasibility and expectations.
What scientific support can CDMOs or CROs provide to biotechs during lead optimization and candidate selection?
BioDuro supports biotechs during lead optimization by contributing to compound design, synthetic route development, biological evaluation, and DMPK profiling. In addition, our platforms with catalyst screening, photochemistry, flow chemistry and library synthesis enablement allows for rapid problem solving to move projects forward.
Single site location for cross-functional collaboration between discovery chemistry, biology, and pharmacokinetics allows for reduced cycle time and timely data generation, allowing for rapid decision-making. This integrated approach, with a single project manager POC, also enables efficient progression from lead optimization to preclinical candidate selection, including the ability to conduct in vivo PK and PK/PD studies when appropriate.
At what stage in drug discovery do you recommend CDMO involvement to ensure smoother downstream development?
Involving a CDMO like BioDuro at the stage of preclinical candidate selection is often beneficial. This is the appropriate time to introduce the compound to the process development and pre-formulation teams, who can begin evaluating manufacturability and formulation strategies. Early involvement helps reduce later-stage risks and supports a smoother handoff into IND-enabling studies.
How do you align your technical team’s input when biotechs bring discovery-stage molecules with limited characterization or formulation data?
When a discovery-stage molecule is introduced with minimal data, BioDuro’s technical teams assess whether additional characterization is needed. Common activities include solubility profiling, salt and polymorph screening, and evaluation of other physical properties. These steps help determine appropriate formulation strategies. Early pre-formulation and formulation screening also enable execution of in vitro and in vivo studies, which are critical at this phase of development.
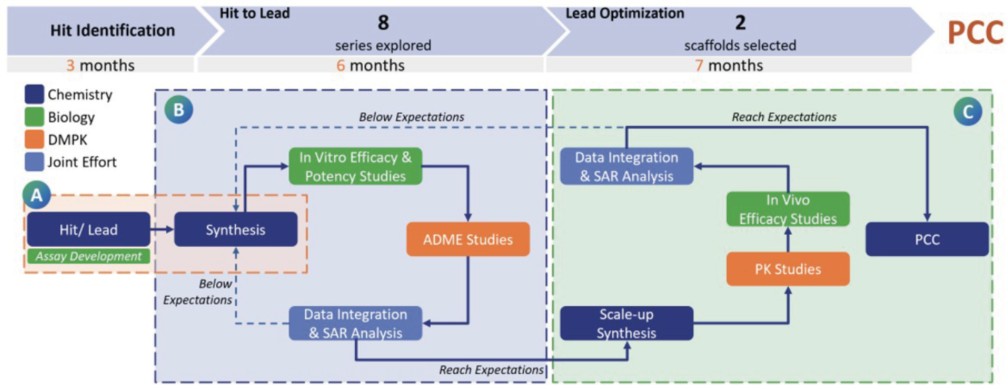
What capabilities have you built to support the transition from hit-to-lead or preclinical discovery to IND-enabling studies?
BioDuro has developed integrated capabilities across discovery chemistry, biology, and DMPK to facilitate a seamless progression from hit validation through to candidate selection. At the point of preclinical candidate nomination, our process and formulation development teams are brought in to begin planning for scale-up and downstream development. This structured handoff allows for continuity across project stages. In one representative program, over 400 compounds were synthesized and tested within 16 months using this integrated workflow.
How do you plan internal resources and capacity when handling multiple biotech clients in parallel without compromising timelines or quality?
BioDuro manages resource planning by assigning dedicated project teams to each biotech partner. These teams include scientific staff, group leaders, and directors who are aligned with project needs and timelines. We have multiple sites for chemistry where clients can locate their teams along with needed analytical teams.
If integrated support across chemistry, biology and DMPK are needed, we have two sites enabled for cross functional collaboration. Each of these sites can support multiple clients with dedicated labs for IP security.
Having a large and experienced scientific workforce allows for flexibility and scalability while maintaining delivery standards across concurrent programs.
How do you communicate timeline risks and mitigation strategies effectively to biotech partners?
Timeline projections at BioDuro are based on scientific assessment and past experience. If risks are identified—such as potential delays from complex chemistry or external shipping issues—clients are informed early, and backup strategies are discussed. Open and ongoing communication is central to this process. The goal is to work collaboratively with biotech/pharma partners to manage uncertainties and maintain alignment throughout the project lifecycle. What practices do you follow to ensure transparency, accountability, and traceability in complex development programs?
BioDuro maintains transparency through regular updates, including weekly meetings, detailed written reports, and ad hoc discussions when needed. Teams operate with a strong sense of ownership and responsibility. All scientific activities are recorded in lab notebooks and electronic systems in accordance with regulatory expectations. Internal QA audits and client inspections are routinely conducted to ensure traceability and compliance throughout the development process.

Sylvia Wojczewski, PhD
CEO, BioSpring GmbH
Partnering With The Right CDMO To Accelerate Clinical & Commercial Development Timelines
How do you approach early-stage discussions with biotechs during RFP or feasibility phases?
At all stages, beginning with the RFP process, communication is key when interacting with our biotech partners. Clear alignment on goals, objectives, and milestones define the success of a partnership. As a CDMO, we are responsible for understanding client and program-specific requirements to ensure we meet their desired timelines, deliver high-quality products and services, stay within budget, and support navigation of complex regulatory landscapes. Part of our role is to advise and guide on strategies tailored to the individual goals of the biopharma client. Early and rigorous planning allows us to work together to scale production and serves as a foundation for successful later stage development.
What scientific support can CDMOs or CROs provide to biotechs during lead optimization and candidate selection? At what stage in drug discovery do you recommend CDMO involvement to ensure smoother downstream development?
Our goal is to engage with our clients as early as possible in the drug development lifecycle. Ideally, supporting them already during their lead candidate screening and optimization stage. During those early stages, it is key to be transparent as a CDMO. Sharing feedback concerning manufacturability of lead candidates as opposed to just delivering a product has helped many clients make informed decisions during their lead candidate selection process and address potential challenges early.
Further, as a CDMO with capabilities spanning from early development through commercialization, we leverage clinical and commercial manufacturing experience when working with our clients to prepare for the next steps, e.g., entering clinical development or additional scale-up and process refinement stages in preparation of commercial supply. Clients trust the proven concepts and expertise we apply consistently across all CMC activities. Considering scalability early on is essential to manage cost and timelines to secure market leadership early in this fast-paced biopharma environment.
As time-to-clinic, or time-to-market, is always crucial due to the increased competitiveness in the biopharmaceutical sector, collaborating closely with the CDMO partner early on helps to save valuable time and money when entering into clinical trials or commercializing a therapy. Working with a CDMO that is highly experienced with the given modality is critical, as this allows the CDMO partner to leverage its existing platform knowledge, ensuring ambitious timelines and tight budgets are met.
What capabilities have you built to support the transition from hit-to-lead or preclinical discovery to IND-enabling studies?
BioSpring takes a comprehensive approach to accelerating clients through the entire drug development lifecycle, with services spanning from manufacturing and regulatory support to analytical/bioanalytical services. We offer high-throughput production of nucleic acids to support discovery, then perform a seamless scale-up of the lead candidates to larger batch sizes for early preclinical work and regulatory toxicity studies. In parallel, we offer bioanalytical assays and a full suite of in-house analytical testing that provide more insight into the metabolic profile and characteristics of the therapeutic candidate. When the client is ready, we move with them through the clinical and commercial milestones, manufacturing cGMP batches and performing release and stability testing for both drug substance and drug product. This entire process is coupled by full regulatory support, such as scientific writing of dossier sections, and an expert panel of scientists from every department involved.
How do you support complex modality onboarding (e.g., oligonucleotides, ADCs, PROTACs) from early-phase biotech companies?
We are a CDMO specialized in manufacturing nucleic acids, with almost 30 years of experience. This experience also includes complex molecules involving customized chemistries and starting materials, and heavily modified and conjugated compounds. When onboarding new molecules and programs, we always aim to leverage existing platform knowledge to reduce onboarding timelines and hence time-to-clinic or time-to-market. We have dedicated teams for certain molecule types that help to ensure efficient knowledge transfer, ensuring flexibility and scalability to meet diverse manufacturing needs.
How do you communicate timeline risks and mitigation strategies effectively to biotech partners? What practices do you follow to ensure transparency, accountability, and traceability in complex development programs?
Timeline risks and any technical challenges that might affect the success of a program need to be communicated imminently. Biopharma clients are under a lot of pressure and need to be aware of any potential risks immediately, considering that there are patients awaiting these life-changing treatments. Therefore, it is important to partner with a CDMO that can ensure full-transparency. We implemented best practices to guarantee full visibility and traceability at every stage of the project. Frequent project team meetings, as well as ad hoc meetings are important to keep all stakeholders informed and to ensure efficient resolution of any hurdles.
How do you support “fast-to-clinic” timelines while maintaining regulatory compliance and phase-appropriate quality?
As a CDMO, we know that heightened regulatory demands can complicate and slow the path to clinic.
Clients are often collaborating with us early on in preparation of regulatory filings for both their clinical and commercial programs. It’s important to clients that we demonstrate a high-level of flexibility and communication to accommodate their goals and tight timelines, as well as have robust workflows and capacities in place to ensure regulatory compliance and quality across all phases. Beyond providing manufacturing and analytical services, we also prepare our clients to be “regulatory-ready”. With our experience spanning almost three decades across a diverse client base, programs, and types of modalities, we are uniquely positioned to support clients with any regulatory questions and help them navigate this complex regulatory landscape while adhering to the highest quality standards.

Andrew Mitchell
Associate VP, Business Development, BIOVECTRA
At what stage in drug discovery do you recommend CDMO involvement to ensure smoother downstream development?
As one of the largest North American biologic microbial fermentation CDMOs with various available scales, we recommend biotech companies engage a CDMO as early as possible, especially if they anticipate potential issues during process development. Ideally, any issues will be identified at the preclinical or, at the latest, by the end of phase 1 clinical trials. With our experience of various projects, this timing allows us to make changes and finalize the process before phase 2, when having a robust and reproducible process is critical. When a biotech approaches CDMOs much later in the cycle, it’s likely going to add cost and involve some rework and timeline delays.
There might be a step that works well at a small scale in the lab — such as the chosen cell line or microbial expression system for a recombinant protein or plasmid — but won’t scale up efficiently for clinical trial production. Even when a startup is keen on getting phase 1 safety data as quickly as possible, knowing that most products won’t make it to the later phases, we proactively flag steps in the process that may cause problems during scale-up.
Another reason to engage early is that we may need to optimize our equipment based on the client’s process or their product. If, for instance, the process has an unusual step requiring a high volume of solvent or aqueous media, we need to confirm that we can handle those requirements or find an alternative solution. Early engagement allows us to match a biotech’s needs with our capacity. If they come to us during later stages of development, we may need to make adjustments to the process train equipment, which always has the potential to cause delays.
What’s your approach to method development and tech transfer when receiving incomplete or early-phase packages from biotechs?
We evaluate the initial package provided by the client for incomplete information. A follow-up meeting with the client and our technical team clarifies outstanding details. Our initial proposal includes assumptions—expected yield, recovery rates, and specific equipment requirements—and then we address any remaining unclear or missing information.
Once we win a proposal, we immediately do a familiarization run to ensure the package works as intended. This familiarization run may tell us we need to do further development work on a part of the process to scale up or, in rare cases, it may even indicate that we can’t go any further.
Each of our development hubs—for biologics, small molecules, and purification—supports tech transfers to manufacturing operations. The process development and manufacturing science and technology teams work in close coordination to minimize scale-up issues as processes are developed from early-phase packages for large-scale production.
What examples or lessons learned can you share from both successful and challenging biotech partnerships?
The success of a partnership depends on transparent communication. As a CDMO, we rely on clients to openly share what they know—as well as what they don’t. On our side, we have to explain any potential issues we foresee. Agreeing on expectations, deliverables, and the scope of work helps form the foundation of a productive collaboration and make a project successful. Once we’ve onboarded a project, the assigned project manager meets with the client every week for a project review and to ensure the collaboration is going smoothly.
Even then, there are ways communication can break down. Initially, we deal with the purchasing department of a biotech company. Once a project is onboarded, we tend to interact with project groups and transfer groups. If they work in isolation from their manufacturing groups, they may not understand the complete picture. When clients aren’t upfront about their challenges and provide vague expectations or targets without clear specifications, it can make it difficult for us to effectively deliver a product. A good example was a tech transfer of a continuous fermentation process with a pull-off on the tank that allowed us to complete downstream processing in parallel. We found the fermentation challenging to complete, prolonging the process. While we collaborated with our client to improve the aseptic processes and solve the problems, we learned they were facing the same issues internally—information that, if shared earlier, could have accelerated our problem-solving.
How do you assess and communicate your ability to support lifecycle development—from early-phase through to commercialization?
Our experience offering complete lifecycle support allows us to guide clients through each development phase. We help them understand how GMP requirements change for each phase of clinical trials as they become progressively more encompassing.
There are times when we assess a project and identify a gap between the client’s long-term needs and our existing capabilities. In these instances, we have a discussion about what’s needed to take their product to the volumes they require. We have had several projects where we’ve successfully built capacity for a client’s specific program.
We are continuously reviewing and assessing our internal capabilities so we can best support clients. A good recent example is the expansion of our process development offerings. We now have a lot more equipment and the ability to support a larger number of projects side-by-side on the journey through the clinic to commercial. We now support clients in the lab from 250 mL to 50 L scales. Large-scale production for clinical material and beyond occurs in a commercial facility with larger equipment, including 100 L and 1,000 L single-use fermenters and 17,000 L stainless steel fermenters.

Kathryn L. Ackley, PhD.
Oligonucleotide CMC Consultant, USA
What are your top 3 criteria when selecting a CDMO for complex modalities like ADCs or oligonucleotides?
My top three criteria include the technical expertise of the CDMO staff, the CDMO’s analytical development capabilities, and the CDMO’s quality management system. The CDMO should have a team of people with experience in the modality of interest. If all the CDMO’s technical knowledge resides within a few people, the project will be vulnerable to staffing changes. Early in the vendor selection process, ask for a video call or on-site visit with the technical team that will oversee the project. The biotech may wish to seek assistance from a knowledgeable consultant to help with the vendor selection process. Consultants can be especially useful when the biotech’s internal staff has limited experience with the modality. Ask to see an organizational chart to verify that the CDMO has adequate staffing levels to support the project.
Complex modalities typically require complex analytical methods. Large molecules such as oligonucleotides often need orthogonal purity methods, multiple identification tests, and characterization of complex impurity mixtures. Significant analytical development resources are needed to support these activities. The success of the manufacturing process development depends upon analytical development. The manufacturing process is established by measuring the impact of changes to process parameters. The speed at which the CDMO can generate quality analytical data to support process development, manufacturing, and product characterization will dictate the pace of the entire project. For this reason, I always pay close attention to the CDMO’s analytical development capabilities.
Finally, the CDMO must have a robust quality system. A drug sponsor never wants to recall a drug due to safety concerns. Many drug sponsors wait to perform a quality audit after the vendor selection process is complete. The quality audit is often viewed as a formality, but the audit will uncover important information that can help with the vendor selection process.
What steps can biotechs take to define project requirements better and avoid unnecessary delays?
The first step a drug sponsor should take is to perform a thorough assessment of the company’s capabilities to identify areas where there may be gaps in expertise or resources. The drug sponsor is ultimately responsible for a drug’s safety. The sponsor may outsource activities such as manufacturing, but the sponsor must still provide sufficient oversight. The drug sponsor can obtain the necessary resources through hiring employees or through retaining consultants or contractors. The CDMO relationship is a partnership, and both parties need to have adequate resources to keep the project on track.
Make sure all the necessary internal stakeholders are involved in the initial project planning. The non-clinical, CMC, and clinical team members should all have the chance to review the project plan and make their respective needs known. Ideally, this review will take place before the RFP is finalized and sent to the CDMO. Project delays can occur when internal stakeholders have not been properly consulted and the project scope must be changed during the execution of the project.
Drug development programs generate many documents that require client review and approval. The CDMO will ask the drug sponsor to review documents such as development reports, manufacturing batch records, analytical protocols, and validation reports. These documents need to be reviewed by the client within a few working days to maintain the project timelines. The drug sponsor should develop an internal process for reviewing and approving documents from the CDMO before the project starts. Doing so will avoid delays.
During initial due diligence, how do you evaluate a CDMO’s quality management system?
The initial RFP should contain the entire scope of work needed from the CDMO including all ancillary activities to support manufacturing. For example, a drug sponsor needing a batch for a Phase I clinical trial might also include the following activities in the RFP: analytical method development, analytical method validation, process development, production of a demonstration batch, preparation and characterization of a reference standard, and stability studies. The RFP should be as detailed as possible and should contain information on novel raw materials or excipients. If the CDMO is in a different country from the drug sponsor, then additional details need to be addressed such as the language to be used in documentation and the currency to be used for payment. The sponsor’s wishes should be expressed in the RFP. The CDMO’s position should be clearly stated in the proposal. Drug sponsors should establish a confidentiality agreement with the CDMO before submitting the RFP. (1)
What should biotechs include in their initial RFPs to better align CDMO capabilities with project expectations?
The initial RFP should contain the entire scope of work needed from the CDMO including all ancillary activities to support manufacturing. For example, a drug sponsor needing a batch for a Phase I clinical trial might also include the following activities in the RFP: analytical method development, analytical method validation, process development, production of a demonstration batch, preparation and characterization of a reference standard, and stability studies. The RFP should be as detailed as possible and should contain information on novel raw materials or excipients. If the CDMO is in a different country from the drug sponsor, then additional details need to be addressed such as the language to be used in documentation and the currency to be used for payment. The sponsor’s wishes should be expressed in the RFP. The CDMO’s position should be clearly stated in the proposal. Drug sponsors should establish a confidentiality agreement with the CDMO before submitting the RFP. (1)
References and notes
- Ackley K, How To Select a Therapeutic Oligonucleotide CDMO, Chem Today, 2023; 41(1): 4-6.

Dr. Stephen Houldsworth
Sr. VP, Global Head of Small Molecules Platform, CordenPharma
Sound CDMO Considerations for Biotechs: Integrated Services, Early-Phase Technologies, Transparent Project Management & Robust Platforms
How do you align your technical team’s input when biotechs bring discovery-stage molecules with limited characterization or formulation data?
It all starts with the customer’s RFP. Our technical teams first explore the customer’s package and put together a proposal which includes core elements that we consider essential to the success of the program. These may be related to the safety of our operators, familiarization of the process, or the quality of the product and compliance with the Good Manufacturing Practices involved – for us these are non-negotiables.
We will then suggest optional elements with a business or scientific rationale as to how they will advance the asset or reduce the risk of the program, either from a manufacturing point of view or a regulatory perspective. We then present the full RFP to the customer, usually with strong suggestions regarding the optional elements which are based on our track record of experience and ability to anticipate their needs – but at the end of the day it is the customer’s decision.
In order to address limited characterization and formulation data, it helps biotechs tremendously to choose a CDMO with early-phase solid-state API and bioavailability enhancement Drug Product services to address these issues early on.
How do you plan internal resources and capacity when handling multiple biotech clients in parallel without compromising timelines or quality?
It is always a juggling act working with multiple biotech clients but our global network of Facilities, Site Project Managers, Technical Teams, Platform Directors and Sales Reps work together seamlessly to coordinate multiple biotech projects in parallel. We recommend that biotechs work with CDMOs that have large enough Process Development and Analytical resources to ensure there is always sufficient bandwidth available to accommodate shifting priorities.
How do you communicate timeline risks and mitigation strategies effectively to biotech partners?
Open communication is key, along with presenting options/solutions as challenges come up. Indicating where points of concern could impact timelines at the outset of a project is an important part of the process, as the customer may actually have valuable information they have not shared previously which can shed light on the situation or explain why they choose a particular path. These details are often crucial to informing a sound strategy.
We often present mitigation strategies up front, but since budget is an ever-present concern for the biotech customer, we usually start with the basic strategy and adjust as needed on the journey. We often find the biotech customers’ least considered aspect of planning centers around the final solid-state form of the API, and how this will impact their drug product aspirations.
Fortunately, CordenPharma has in-house Centres of Excellence for API Solid-State and Drug Product Bioavailability Enhancement considerations that address these decisions when the need arises. We encourage customers to take advantage of these services up front during development so we can maintain timelines, but we also involve the group later as challenges begin to transpire during drug product development and production.
What practices do you follow to ensure transparency, accountability, and traceability in complex development programs?
Utilizing our Smartsheet project management system, we have created a one-stop portal for all project-related details, where customers can follow along with the project progress in real time and find all project meeting minutes, action item lists, documentation trackers and of course, the all-important timeline, stored in one place.
Through this tool the customer has the most transparent access possible to their project within our systems. When multiple sites within our integrated network are involved to execute both Drug Substance and Drug Product manufacturing via our integrated supply offering, or when customers take advantage of our Centres of Excellence to solve potentially complex issues, the process can become very complicated from a project management perspective.
So here, our Global Project Management systems become extremely beneficial to biotechs by creating one common point of contact. Having that one central resource to coordinate the activities between multiple sites and priorities relieves them of a significant burden. It also creates one common vision for the project internally, which is obviously aligned with the customers’ vision.
What tools or digital platforms (e.g., dashboards, LIMS, collaborative portals) have improved real-time visibility and engagement with biotech clients?
The implementation of our common global Smartsheet Project Management system provides our customers with the most efficient access possible to our systems to track and monitor the progress of their project. Updated in real time, the system provides a common, one-stop-shop approach to project updates, project meeting minutes, timeline updates, documentation trackers, upcoming milestones and action logs. The system is obviously protected so that only the customer can securely view the project externally, but internally the full project team has access to update it as the latest information becomes available.
What’s your approach to method development and tech transfer when receiving incomplete or early-phase packages from biotechs?
When we sign on a new client, we do so on the understanding that we are here to provide whatever expert CDMO advice possible, since it’s in both parties’ interest that the program is successful. In many cases, this may be a partner’s first or second asset to be taken into the clinic, whereas in our case, we have decades of experience helping biotechs with incomplete or early-phase packages find effective method development to bring their projects through clinical phases. Some clients take advantage of that expertise, which an experienced CDMO should offer freely, and in other cases they may decide to hire CMC experts – so often a working partnership gets formed to help shepherd the asset into the clinic.
An important aspect of successful tech transfer to consider when selecting a CDMO is their facility network. Does it provide seamlessly integrated, global services that alleviate distractions and potential roadblocks to successfully meeting timelines?
LISTEN TO OUR PODCAST
Subscribe and get free unlimited access!
15K articles and news from our archive.
Trusted by








40 years connecting the world of science for industry
Our journals:





